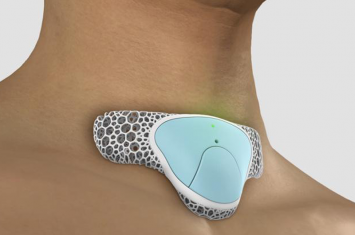
Princeton Medical Review: Continuous Vital-Sign Monitoring: Preventing Opioid-Induced Respiratory Depression
September, 2025 article by Anthony Rice, Princeton Medical Review
Read More