Hypertension (High Blood Pressure) is the most frequent modifiable risk factor for cardiovascular disease affecting 30 to 40% of the adult population in the world (NIH.gov), including about half of US adults.
Heart disease is the world’s #1 killer, contributing to 1 of every 3 deaths.
In the US alone (CDC statistics)
- 1000 die suddenly each day due to heart attack or stroke
- 800,000 heart attacks each year; 25% are repeat events
- 790,000 strokes each year; 23% are repeat events
- 22% die within a year of the first heart attack
- 40 million have uncontrolled high blood pressure
- 4x more likely to die from stroke; 3x more likely to die from heart attack
- Over half of heart attacks are “silent”
Most heart attacks and strokes are due to uncontrolled hypertension: many are preventable. According to the American Heart Association, the key to controlling hypertension is monitoring, which today, cannot be adequately done outside of a hospital.
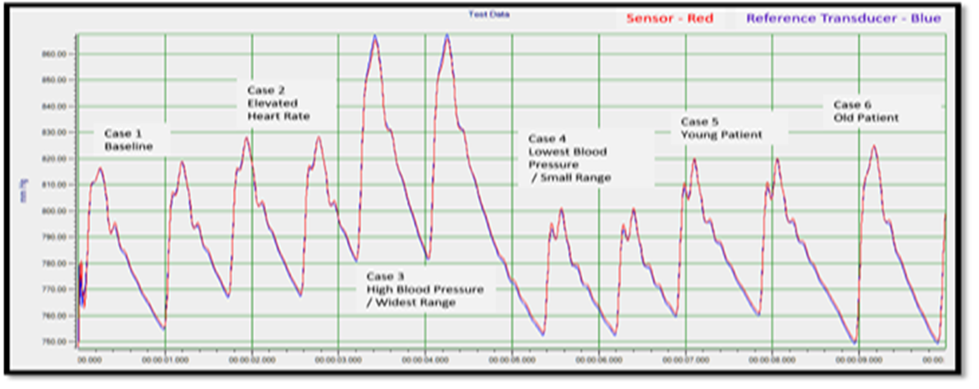
Continuous monitoring and data insights from the blood pressure waveform can detect and may prevent adverse cardiac events, heart failure, and renal degradation years in advance.
The RTM Cardiac Monitoring System features a long-term, outpatient-patient-implantable blood pressure sensor which accurately and continuously measures the entire arterial blood pressure waveform, and electrocardiogram. Sensor data feeds predictive algorithms to improve diagnostics and medication dosing related to hypertension and other cardiac issues, ultimately leading to prediction and prevention of adverse cardiac events. Relevant data can be sent to a smart device, or a central monitoring station for advanced analysis by machine learning (AI) computer algorithms and a clinician.
Hypertension and Blood Pressure variability significantly increase the risk for adverse cardiovascular events; real-time monitoring with our sensor will enable not only timely detection of significant changes in physiology with alerts and alarms, but also a robust database of BP and vital sign trend data obtained from hundreds of ambulatory patients that will be used to optimize models of cardiovascular risk in relation to diet, exercise, medications, devices, and surgical interventions – crucially important information not only for patients, but also for public health officials and for life, disability, and health insurance companies. This clinically relevant trend data does not currently exist.
Real-time monitoring could reduce the incidence and severity of acute and chronic cardiovascular adverse events and transform the way physicians practice clinical medicine in the outpatient setting.
Our prototype long-term implantable Cardiac Monitoring System (CMS) has been tested in-vitro and in-vivo in anesthetized year-long studies in canines to demonstrate the feasibility and optimize the implantation process. The RTM CMS replicated in-hospital monitor functionality in the in-vivo trials without stationary hardware and wires.
RTM has 13 issued patents covering the device and functionality of the CMS.